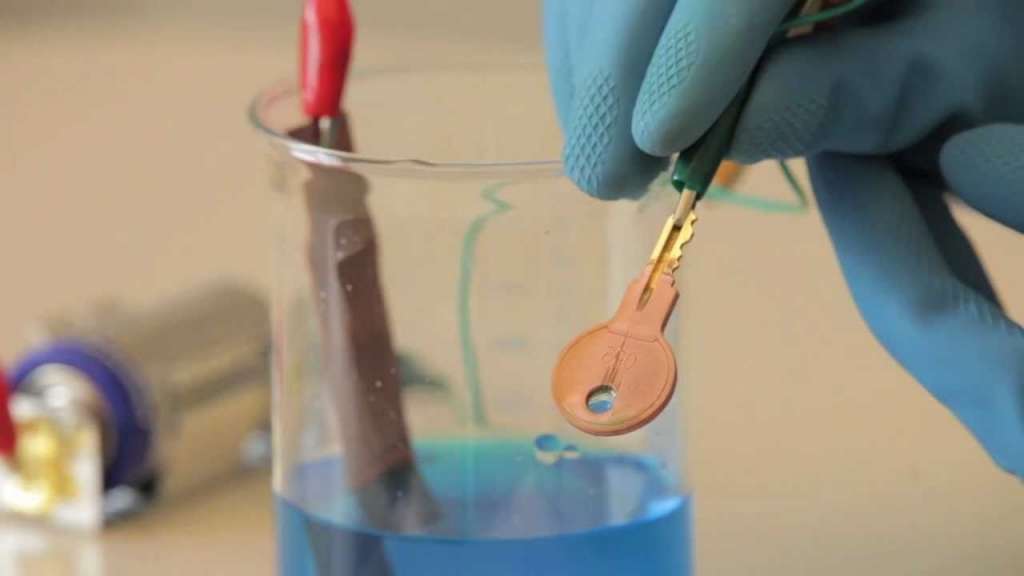
Electroplating is the process of applying a metal coating on another piece of metal (or another conductive surface) through an electro deposition process. In electroplating, the deposited metal becomes part of the existing product with the plating/coating. In Electroplating, both an anode and a cathode (the metal part to be coated) are immersed in an electrolytic bath that is composed of a solution of salts, including the metal to be plated. A direct current (DC) of electricity is passed through the solution, effecting the transfer of metal ions onto the cathodic surface, plating the metal onto the item.